 AFP via Getty Images
AFP via Getty Images
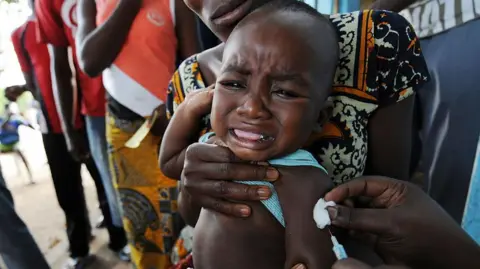
The $1.6m (£1.2m) trial was funded by the US Centers for Disease Control and Prevention (stock image)
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticised by the World Health Organization as "unethical".
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age.
The WHO said it had "significant concerns" about the plan, and described the birth-dose vaccine as "an effective and essential public health intervention, with a proven record".
The US health department, headed by Robert F Kennedy Jr, who has questioned the effects of vaccines, had sought to use the trial to answer questions about the jab's broader health effects.
The WHO said on Friday that its concerns regarded the study's scientific justification, ethical safeguards and consistency with established standards for research involving humans.
It stressed that the jab had been used for more than three decades in more than 115 countries.
The WHO said giving a proven life-saving intervention to some newborns but not others exposed them to "potentially irreversible harm".
A sizeable portion of Guinea-Bissau's population is estimated to have hepatitis B, and the WHO says vaccination at birth prevents the virus being transmitted from mother to baby in 70-95% of cases.
It argued that trials giving one group a placebo or not treating them were only acceptable when no proven treatment existed, something that was not the case with the hepatitis B birth dose vaccine.
The WHO recommends that all newborns receive the hepatitis B vaccine within 24 hours of birth. It says infection at birth is the most common way of having a lifelong infection, with 90% of infected newborns becoming chronic carriers.
In Guinea-Bissau, the dose is currently given at six weeks, though authorities plan to introduce the birth dose nationwide by 2028 to align with global standards, something the WHO said it would help accelerate.
A total of 14,000 babies in the West African country were due to be involved in the study funded by the US and led by Danish researchers.
But public outrage at the project prompted the Guinea-Bissau government to suspend it last month.
Critics have questioned why babies in the African country were being proposed for the trial.
Two months ago, a panel of top advisers voted to stop recommending that all newborns in the US receive a hepatitis B vaccine.
Kennedy has on several occasions denied being against vaccinations and has said he and his children had been vaccinated, but has also repeatedly stated widely debunked claims about vaccine harms.
Vocal opponents of the project in Guinea-Bissau include the country's former health minister, Magda Robalo.
Most people with the virus do not have any symptoms, or have very mild symptoms.
But some people can experience jaundice, dark urine, feeling very tired, nausea, vomiting and pain in the abdomen.
The WHO says chronic patients have a high risk of cirrhosis and liver cancer.
You may also be interested in:

 Getty Images/BBC
Getty Images/BBC

 1 hour ago
2
1 hour ago
2